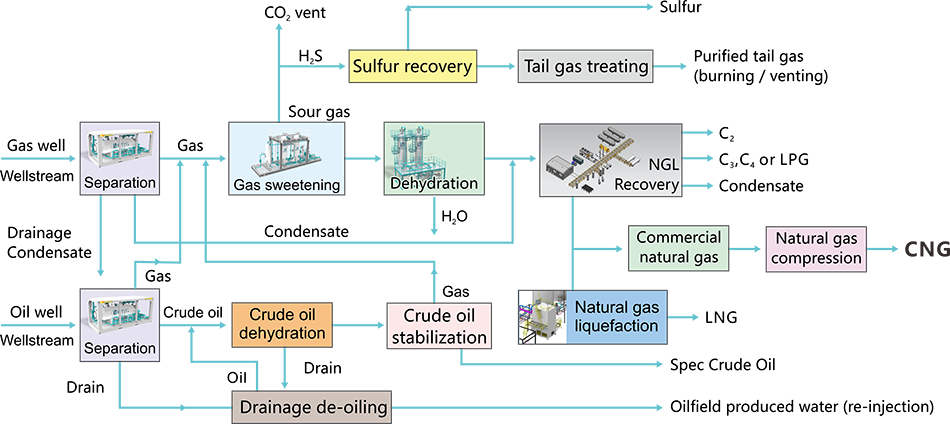
Natural Gas Purification (Processing) and Recovery

Separation

Gas Sweetening Unit

Natural Gas Dehydration Unit
NGL Recovery Unit

Separation
Gas Sweetening Unit
-
-
The Importance of Natural Gas Desulfurization
The acidic components such as H2S, CO2 and organic sulfur contained in natural gas will corrode metals in the presence of water; Sulfur containing components have the disadvantages of unpleasant odor, high toxicity, poisoning catalyst, etc. CO2 is a non-combustible gas, which not only affects the calorific value of natural gas, but also affects the efficiency of pipeline transportation. In particular, H2S is a gas with unpleasant rotten egg smell and great toxicity. If the content of H2S in the air reaches tens of mg/m3, it will cause tears and headaches. High concentration of hydrogen sulfide is life-threatening to people; H2S will severely corrode equipment and pipelines in the presence of water and at high temperatures (above 400 ℃); It also causes hydrogen embrittlement to some steels, and valve rod fracture and valve plate falling off in natural gas purification plants. Organic sulfur poisoning will cause nausea, vomiting and other symptoms, and may even cause heart failure, respiratory paralysis and death.
Therefore, natural gas desulfurization has the benefits of protecting the environment, protecting equipment, pipelines and instruments from corrosion and facilitating the use of downstream users.
At the same time, it can also turn harm for benefit and recycle resources. After the separation of hydrogen sulfide in natural gas, sulfur (bright yellow, purity up to 99.9) is made by Claus reaction. It can produce sulfur and sulfur-containing products, which are widely used in industry, agriculture and other fields.
The high-purity CO2 separated from natural gas with high content of CO2 can be used to make dry ice, and can also be used for re-injection formation on oil production to improve oil recovery.
-

-
Techniques for Desulphurization & Decarbonization of Natural Gas
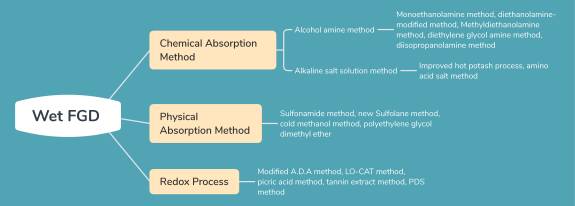
As for the removal of acid gas from natural gas, a number of techniques have been developed, which can be divided into wet and dry methods. Currently, dry desulfurization is rarely used in industry, and large-scale industrial units mainly adopt wet desulfurization. According to the absorption and regeneration methods of solution, wet desulfurization can be divided into chemical absorption, physical absorption and oxidation-reduction.
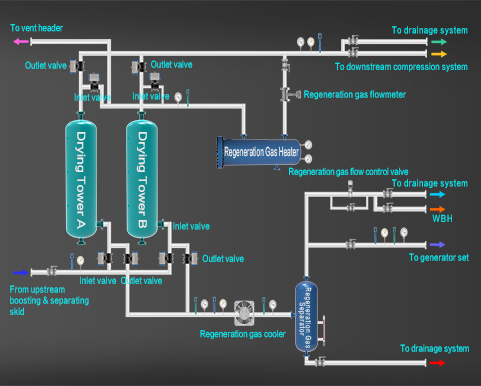
Gas Dehydration Unit
-
-
The Importance of Natural Gas Dehydration
The natural gas will be gradually cooled in the gas inlet and transportation pipeline, and the saturated steam in the natural gas will gradually precipitate to form water and condensate liquid. The liquid flows with natural gas and accumulates at the lower part of the pipeline, resulting in increased resistance. When the liquid accumulates to form slugs, its flow has great inertia, which will cause damage to the liquid catcher of the separator at the end of the pipeline.
The existence of liquid in the pipeline will reduce the transportation capacity of the pipeline.
Water and other liquids in pipelines as well as hydrogen sulfide and carbon dioxide in natural gas form corrosive liquid, which causes corrosion in the pipeline, shortens the service life of the pipeline, and increases the frequency of pipeline bursts.
Water is easy to form hydrate in the pipeline, which blocks the pipeline and affects normal production. In order to protect the long-distance natural gas pipeline and improve the pipeline transportation efficiency, natural gas must be dehydrated before entering the gas pipeline.
-
-
Techniques & Features of Natural Gas Dehydration
There are various techniques for natural gas dehydration. According to its principle, it can be divided into three categories: freezing and separation, solid adsorption drying and solvent absorption. Changqing Natural Gas Purification Plant adopts the triethylene glycol (TEG) dehydration process. The molecular formula of TEG is HO(CH2)2O·(CH2)2O·(CH2)2OH. TEG has the characteristics of strong water absorption and easy regeneration under high temperature, which can be used as a dehydrating agent to reduce the water content in natural gas. The dehydration process of TEG is a physical process, which uses the strong water absorption of TEG to absorb water in natural gas, and the TEG that absorbs water is called rich liquid; After the rich liquid enters the reboiler, the water is evaporated under normal pressure and high temperature. Coupled with dry gas stripping, a TEG lean liquid with a concentration of more than 99% can be obtained, which can be recycled. This process has the following characteristics:
Simple process flow, sophisticated technology, large dew point drop (30~60℃), excellent thermal stability, easy regeneration, less loss, low investment and operation costs, etc.
Setting the lean liquid cooling prior to the inlet of the circulating pump not only improves the operating conditions of the circulating pump, but also reduces the temperature of the product gas and reduces the impact on the pipeline transportation capacity of long-distance pipelines.
Set a filter on the rich liquid pipeline to remove the mechanical impurities and degradation products carried in the solution system, keep the solution clean, and facilitate the long-term operation of the unit.
A medium pressure steam system specifically designed for TEG regeneration can be omitted.
Sulfur Recovery Unit
-

-
Sulfur Recovery Unit
The sulfur recovery unit is a supporting environmental protection project for the desulfurization unit, which mainly converts the acid gas removed by the desulfurization unit into sulfur. The tail gas is then incinerated in the acid gas incineration unit and is being discharge into the atmosphere through the chimney.
The core unit (reaction unit) is a series of continuous production, and the auxiliary unit (sulfur forming unit) is intermittent production.
The main production units include: sulfur recovery unit, sulfur molding and packaging, and sulfur warehouse.
The sulfur recovery unit of the first purification plant has a processing capacity of 10-27×104 m³/d of acid gas, of which the H2S content is 1.3-3.4% (mol).
The sulfur recovery unit of the second purification plant has a processing capacity of 12-30×104 m³/d of acid gas, of which the H2S content is 1.55-3.59% (mol).
Both units adopt the Clinsulf-DO direct oxidation process of Linde Company, Germany.
-

-
Brief Description of Sulfur Recovery Process
The acid gas from the air and desulfurization unit is preheated to 200 ℃ by medium pressure steam according to a certain proportion (the amount of air in the ideal proportion is calculated by one molecule of O2 and 1/2, and the proportion of air and H2S is generally 0.42). After entering the reactor, the hydrogen sulfide and oxygen in the acid gas undergo an exothermic reaction under the action of the catalyst to generate elemental sulfur. The normal temperature of the reactor is 292.1 ℃ (this temperature changes with the change of H2S content, and the control basis is shown in the table below). After the sulfur vapor from the reactor passes through the sulfur condenser and sulfur separator, the liquid sulfur enters the liquid sulfur storage tank, and then the liquid sulfur is transported to the sulfur solidification condenser by the liquid sulfur pump, and evenly drips onto the rotating steel belt through the distributor. Under the action of cooling water, the liquid sulfur solidifies on the steel belt into hemispherical particles, which are collected in the packaging hopper, and the tail gas is then incinerated before being discharge.
-

-
Acid Gas Incineration
The acid gas incineration unit is mainly used to incinerate the acid gas removed by the desulfurization unit, oxidize the acid gas H2S into SO2, and then discharge it into the environment. After the sulfur recovery unit is completed, this unit mainly deals with the tail gas generated by the sulfur recovery unit.
The acid gas removed by the desulfurization unit enters the sulfur recovery unit for processing, and the resulting tail gas enters the unit and is incinerated in the negative pressure incinerator (H-2101), so that H2S and sulfur in the tail gas are oxidized into SO2 and then discharged into the atmosphere. The high-temperature flue gas from the incinerator enters the chimney and is discharged.
In order to ensure safety, flame arresters are installed in the acid gas inlet and fuel gas inlet pipes. During normal operation, the furnace temperature should be controlled at about 600 ℃, and the furnace temperature and fuel gas pressure should be controlled in cascade. The purpose of controlling the furnace temperature is achieved by adjusting the fuel pressure.
NGL Recovery Unit
-

-
NGL Recovery Unit
In addition to methane, natural gas (especially condensate gas and associated gas) generally contains a certain amount of ethane, propane, butane, pentane and heavier hydrocarbons. In order to meet the quality index of commercial natural gas or the quality requirements for hydrocarbon dew point of pipeline gas, or to obtain valuable liquid fuels and chemical raw materials, it is necessary to separate and recover hydrocarbons in natural gas according to certain requirements.
Currently, ethane, propane, butane, pentane and heavier hydrocarbons in natural gas are recovered in liquid form except ethane which is sometimes recovered in gas form. The liquid hydrocarbon mixture recovered from natural gas is called natural gas liquid (NGL).The composition of NGL varies according to the composition of natural gas, the purpose and methods of NGL recovery. The process of recovering condensate from natural gas is called NGL recovery, The recovered NGL is either directly used as a commodity or further separated into ethane, liquefied petroleum gas (LPG, which can be propane, butane or a mixture of propane and butane) and natural gasoline (C5+) according to relevant product quality indicators. Therefore, NGL recovery generally includes natural gas separation process.
-

-
The Purpose of NGL Recovery
The Reason of Natural Gas Condensate (NGL) Recovery
- Dew point control
- Maximizing operating profits
- NGLs are used as petrochemical feedstock
- Condensate stabilization
-

-
NGL Recovery Techniques
- Adsorption
- Absorption
- Refrigeration
- Recontacting-compression
- Combination of the several methods
Liquefied Natural Gas (LNG)
-

-
Advantages of LNG
Since the volume of LNG is about 1/625 of the volume of gas before liquefaction, it is conducive to storage and transportation.
LNG can not only be used as a clean alternative fuel for gasoline and diesel, but also can be used to produce methanol, ammonia and other chemical products. In addition, LNG has been widely used in gas peak regulation and emergency gas sources, improving the stability of gas supply for urban residents and industrial users. The evaporation phase change during LNG regasification (about 510kJ/kg at -161.5°C) can also be used in cryogenics, refrigeration and other industries.
-
-
LNG Production Process
LNG production generally includes natural gas pretreatment, liquefaction and storage, of which the liquefaction system is the core. Generally, the natural gas is pretreated to remove the components unfavorable to the liquefaction process, and then enters the high-efficiency low-temperature heat exchanger in the liquefaction part to continuously cool down and separate out the heavy hydrocarbons. Finally, the temperature is reduced to -162 ℃ (or slightly higher temperature) under normal pressure (or slightly higher pressure), so as to obtain LNG products, which can be stored, shipped and used under normal pressure (or slightly higher pressure).
-
-
Feed Gas Pretreatment
Feed Gas PretreatmentThe feed gas of LNG plant comes from conventional natural gas, such as gas reservoir gas, condensate gas and associated gas in oil and gas fields, as well as unconventional natural gas, such as coalbed methane, which generally contain H2S, CO2, organic sulfur, heavy hydrocarbons, water vapor, mercury and other impurities to varying degrees. Thus, it is necessary to carry out pretreatment before liquefaction. Feed gas pretreatment process includes:
- 1.Gas Sweetening
- 2.Gas Dehydration
- 3.Mercury Removal
- 4.NGL Recovery
- 5.Nitrogen and Oxygen Removal
-

-
Gas Liquefaction and Refrigeration
In the pretreatment section, acidic gases (H2S and CO2), water, mercury, concentrations of heavier hydrocarbons and any other impurities are either reduced or removed.
There are three main types of liquefaction cycles: cascade, mixed refrigerant, and expansion cycles. Most commercially available liquefaction processes are based on these cycles or a combination of these cycles.
-

-
LNG Storage & Transportation
LNG occupies about 1/600th of its gaseous volume at standard conditions. Above -113 deg. C, methane vapor is lighter than air. The flammable range for methane is 5–15% by volume in air, a range that is wider than for most other gaseous fuels. In addition, the lower flammable limit (5%) and the ignition temperature (632 deg. C) are higher than for other fuels.
The process design of storage tanks of LNG and refrigerants shall comply with requirements under API, NFPA, or other specific relevant standards. Vol. capacity, the purpose of storage, LNG properties, operating conditions, barometric data, external loading, and evaporation rate shall be determined for design. Most LNG is transported by tankers, known as LNG carriers (large, onboard, super-cooled (cryogenic) tanks) and ISO-compliant containers that are suitable for transportation by ships and by trucks.
Compressed Natural Gas (CNG)
-

-
Advantages of CNG
Under normal temperature and high pressure (20~25MPa), the quality of natural gas with the same volume is about 270~300 times larger than that under high pressure conditions, which can greatly improve the storage and transportation volume of natural gas and make the utilization of natural gas more convenient. Nowadays, CNG is widely used in transportation, urban gas and industrial production.
Currently, both at home and abroad are vigorously developing alternative automotive vehicle fuels, and the practical applications include compressed natural gas (CNG), liquefied natural gas (LNG), liquefied petroleum gas (LPG), methanol, ethanol and electric energy.
-

-
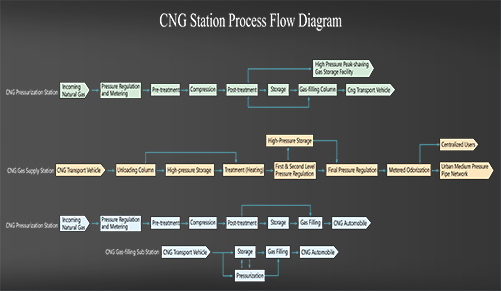
Classification of CNG Station
At present, the classification of CNG stations is not unified yet. According to the purpose of gas supply, it can be generally divided into pressurization station, gas supply station and gas filling station; According to the function setting, it can be divided into single-function station, dual-function station and multi-function station; According to different affiliation, it can be divided into independent station and chain station.
-
-
CNG Station Process
The basic functions of CNG station are natural gas receiving (incoming pressure regulation and metering), processing, compression, supply (including storage, gas filling supply and decompression supply), etc. LPG and LNG stations are usually included, but the in-station process flow is different from that in CNG stations. According to the different gas supply purposes of CNG stations, the following are the process flow diagrams of various CNG stations:
